Introduction
I list relevant sections from Cheung P. C. K., Mehta, B. (Editors), 2015, Handbook of Food Chemistry published by Springer and a few other good articles on the subject. I insert into the relevant sections from the Handbook of Food Chemistry, a section dealing with “Intramuscular Connective Tissue in Muscle Function” by Purslow (2020). My review relates to observed loss in structure if meat is comminuted beyond a certain micron. Under the heading of Mechanically Recovered Meat and Enhanced Meat and Comminuted and Reformed Fresh Meat Products from the Handbook of Food Chemistry, I highlight the relevant discussions which directly speaks to the issue of particle size and therefore cell structure damage in finely comminuted meat products. Those who are interested in the subject of particle size in finely comminuted meat products and size reduction technology only have to scan over the work of Purslow (2020) and see the inherent contribution to structure by the endomysium and perimysium and the sections I highlighted in orange from the Handbook of Food Chemistry.
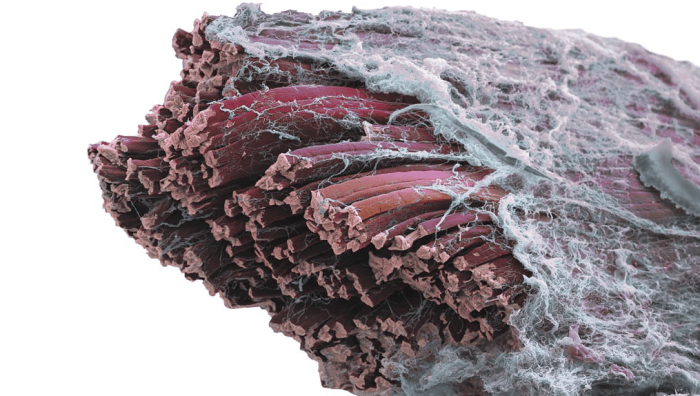
Muscle Structure
Muscle cells, called muscle fibers, are long, narrow, very specialized cells covered by the cell membrane (sarcolemma), whose cytoplasm (sarcoplasm) contains the organelles and the structures responsible of muscle contraction, the myofibrils. Myofibrils are cylindrical structures made up of repeated units known as sarcomeres that cause the striated appearance of muscle when meat is observed by electron microscopy. Striations result from the complex organization of the myofibrillar proteins, responsible for the muscle contraction. The striations originate from alternating dense bands (A-bands) and less dense I-bands; the latter are divided in two parts by dark lines known as Z-lines. The space between two Z-lines is a sarcomere (Huff Lonergan et al. 2010). (Cheung & Mehta, 2015)
“Muscle fibers also contain proteins in the sarcoplasm (sarcoplasmic proteins) and enzymes in different cell structures or organelles (Lawrie 1998; Devine and Chrystall 2000; Huff Lonergan et al. 2010). Connective tissue structures define the organization of muscle. Muscle fibers are individually surrounded by a thin connective tissue sheath (the endomysium) and are bundled into groups or fascicles by connective tissue septa, the perimysium, associated to blood vessels and nerves; the muscle is completely surrounded by the epimysium (Lawrie 1998; Devine and Chrystall 2000). The types and characteristics of muscle fiber and connective tissue proteins are described in the next sections.” (Cheung & Mehta, 2015) Myoglobin and haemaglobin are examples of sarcoplasmic proteins.
“As muscle is converted to meat, the muscle fibers modify their metabolism in order to adapt to the new conditions (absence of oxygen and nutrient supply, lack of residues removing), and as a consequence, many changes occur. The available energy is gradually depleted and the metabolism displaces from aerobic to anaerobic, less efficient in energy [adenosine triphosphate (ATP)] generation, favoring lactic acid production. Its accumulation results in the decrease of tissue pH from values near neutrality to 5.4–5.8. The ionic strength rises, partially due to the inability of ATP-dependent calcium, potassium, and sodium pumps to function. The fibers become less capable of maintaining reducing conditions. All of these alterations have deep effects on muscle proteins and also on proteolytic enzyme systems (Huff Lonergan et al. 2010). Rigor mortis occurs when the level of ATP declines and its concentration is too low to maintain muscle relaxation. After a variable period of time, the resolution of rigor mortis happens, with a progressive softening of the muscles due to the action of proteolytic enzymes (Warriss 2000).” (Cheung & Mehta, 2015)
There are three types of muscles namely skeletal, cardiac and smooth.
Skeletal and smooth muscle cells are elongated, called muscle fibers and cardiac muscle cells are not.
Smooth Muscles
Smooth muscle tissue occurs in the hollow visceral organs, such as the stomach, urinary bladder, and respiratory passages. They force and other substances like food through internal body channels. They have elongated cells, like skeletal muscles, but no striation. Like cardio muscles, they are involuntary.

Another example of a smooth muscle from Michigan Histology and Vistual Microscopy.

Sketelat Muscle
Skeletal muscle fibers are the longest muscle cells and have striations or stripes. Despite the fact that some movement is due to reflex, they are all referred to as voluntary muscles.

Zoomed in on Skeletal Muscle

Cardiac Muscle
Cardiac muscle tissue is found only in the heart. It makes out the bulk of the heart walls. It is not voluntary.
Zoomed in on the cardiac muscle.

Meat Components
Meat is composed of approximately 72–75 % water, 21 % nitrogenous compounds (19 % proteins and 1.5 % nonprotein nitrogen compounds which include nucleotides, peptides, creatine, and creatinine), 2.5–5 % lipids, 1 % non-nitrogenous compounds (vitamins) and carbohydrates (a very small amount of glycogen, transformed into lactic acid during postmortem period), and 1 % ash (potassium, phosphorus, sodium, chlorine, magnesium, calcium, and iron). The most variable compounds are lipids, with values that can vary between 1 % and 15 % (Keeton and Eddy 2004; Kauffman 2012). Meat composition is variable due to the influence of several factors: animal species, breed, sex, feeding, muscle, etc. Their influence is described in section “Factors that Influence Meat Composition.” (Cheung & Mehta, 2015)
Proteins and Other Nitrogen Compounds
“Meat proteins can be divided into three classes: myofibrillar (responsible for contraction-relaxation of muscle and soluble in concentrated salt solutions), sarcoplasmic (metabolic and soluble in water or diluted salt solutions), and connective tissue (support and insoluble) proteins (Tornberg 2005).” (Cheung & Mehta, 2015)
Myofibrillar Proteins

The most abundant myofibrillar protein is myosin; thick filament is made of hundreds of molecules of this protein. Myosin molecule is an oligomer of 1,500 Å in length (1000Å = 1nm) and a molecular weight (MW) of about 500,000 and consists of two identical units with a pair of identical heavy chains with a MW of 200,000 each and two pairs of nonidentical light chains of 16,000 and 20,000. These protein chains form a long tail (double-stranded α-helical rod), a collar (a region between two flexible regions), and two globular heads. These heads have enzymatic activity (ATPase): they can hydrolyze ATP during muscle contraction and provide energy for myosin bound to actin to swivel and pull the thin filaments toward the center of the sarcomere (Huff Lonergan et al. 2010). Trypsin digestion of myosin yields the heavy (MW about 340,000) and light (MW 150,000) meromyosin fragments that correspond to the globular head and to the helical rod, respectively. Heavy meromyosin digestion with papain releases two fragments, S-1, with ATPase activity, and S-2, with fibrous structure, without ATPase activity and unable to bind to F-actin (Lawrie 1998; Murray 2003; Kerth 2013a). Myosin possesses high contents of glutamic and aspartic acids and of dibasic amino acids that make it a highly charged molecule with affinity for calcium and magnesium ions (Lawrie 1998).” (Cheung & Mehta, 2015)
“Actin is the main structural component of the thin filament; each thin filament is composed of two actin filaments turned around themselves, to form a helical strand. Fibrous actin (F-actin) is composed of two chains of globular monomers (G-actin) that consists of 375 amino acids and with a MW of 43,000–47,000; in the presence of Mg2+, G-actin polymerizes noncovalently to form F-actin. Each monomer also possesses a region for binding myosin (Murray 2003; Kerth 2013b).” (Cheung & Mehta, 2015)
“The propound mechanism for muscle contraction involves the use of the energy from ATP hydrolysis for activating conformational changes in myosin head. The myosin head binds actin and forms an actomyosin complex. The ATPase activity of myosin head hydrolyzes ATP producing adenosine diphosphate (ADP) and inorganic phosphorus (Pi), and myosin head suffers a conformational change when ADP leaves it. That provokes the stroke of muscle contraction, pulling the thin filaments toward the center of the sarcomere. This shortens the myofibril and, at a greater scale, the muscle fiber and the complete muscle. Another ATP molecule is necessary for actomyosin dissociation during relaxation causing the myosin head to tilt back to its initial position (Murray 2003; Kerth 2013b).” (Cheung & Mehta, 2015)
“In rigor mortis development, the proteins that constitute the thick and thin filaments, myosin and actin, combine irreversibly to form actomyosin due to the lack of available ATP, and the muscle remains shrunk permanently. In rigor mortis resolution, the myofibrils are fragmented by proteolytic enzymes, and the breakdown of the attachments of thin filaments to the Z-line is observed; however, the thin and thick filaments remain joined. Besides, the intramuscular connective tissue weakens due to some cleave of collagen cross-links (Warriss 2000; Huff Lonergan et al. 2010).” (Cheung & Mehta, 2015)
“Troponin and tropomyosin are two regulatory proteins that play important role in muscle contraction and relaxation and can also be found on the thin filament. Tropomyosin is a long, fibrous protein with two strands with α-helix structure wound around one another and a molecular mass of about 66,000. Tropomyosin covers the myosin binding sites on F-actin when the muscle is in a relaxed state. Troponin consists of three polypeptides or subunits, T, I, and C, with specific functions in muscle contraction: troponin T attaches tropomyosin; troponin I inhibits F-actin-myosin interaction; troponin C is a calcium-binding polypeptide. Both troponin T and I join the three components (Murray 2003; Kerth 2013a).” (Cheung & Mehta, 2015)
The myofilaments are maintained in their positions by a cytoskeleton composed of several proteins. Titin, nebulin, and desmin are proteins located in the Z-line region (between either the thick filament and the z-disk or the thin filament and the M-line) that serve as bridges between the thick and thin filaments of adjacent sarcomeres (Toldrá and Reig 2012; Kerth 2013a). Other elements contribute to the structure and functioning of sarcomeres and muscles, the intermediate and the costameric filaments. The intermediate filaments run perpendicular to the length of muscle fiber, keeping the three-dimensional shape of contractile structure. These proteins are, among others, skelemin, desmin, paranemin, and synemin, and their degradation during postmortem aging can play an important role in tenderness development. The costameric filaments keep the transversal structure organization of muscle fibers, interacting directly with the cell wall and probably also with the endomysium layer outside the sarcolemma. Filamin, dystrophin, talin, and vinculin are proteins of this group and seem to have a role in postmortem degradation of muscles and in the development of meat tenderness (Kerth 2013a).” (Cheung & Mehta, 2015)
“During postmortem aging, the oxidation of myofibrillar proteins occurs, in which some amino acid residues, including histidine, are converted into carbonyl derivatives and can cause the formation of intra- and inter-protein disulfide cross-links. The reactions affect peptide backbone and functional groups in the side chain of amino acid residues (cysteine, methionine, tryptophan, lysine, threonine, arginine, proline). Several factors influence the rate of postmortem oxidation: antemortem factors (breed, diet, rearing system, antemortem stress), type of muscle, and handling of carcasses. These changes produce protein denaturation and loss of protein functionality, affecting water holding capacity, texture, flavor, and nutritional value.” (Cheung & Mehta, 2015)
“Potential initiators of protein oxidation include radical (superoxide, hydroperoxyl, hydroxyl) and non-radical (hydrogen peroxide, hydroperoxides) species. Natural muscle tissue components such as unsaturated fatty acids, heme pigments, metals, and oxidative enzymes are potential precursors or can catalyze the formation of reactive oxygen species during the conversion of muscle to meat and meat aging. Acidification and chilling storage enhance oxidation; high H+concentrations favor prooxidant potential of myoglobin, and acidity affects protein susceptibility to oxidation due to its effects on protein structure and aggregation. Besides, alteration of cellular compartmentalization, release of free catalytic iron and oxidizing enzymes, and lipid oxidation reactions promote the formation of protein carbonyls (Estevez 2011).” (Cheung & Mehta, 2015)
Sarcoplasmic Proteins
“Sarcoplasmic proteins are found in the sarcoplasm and include oxidative enzymes, heme pigments (mainly myoglobin), mitochondrial oxidative enzymes, lysosomal enzymes, and nucleoproteins. Sarcoplasmic proteins are good emulsifiers, but less effective than myofibrillar proteins (Keeton and Eddy 2004). Myoglobin (MW 16,000) is the main sarcoplasmic protein and is responsible for the color of meat. It is a globular protein, globin, consisting of 153 amino acids, bound to the iron atom of a prosthetic heme group (iron(II) protoporphyrin-IX complex) by a histidyl residue of the protein. The heme iron atom can exist in the ferrous (+2) or the ferric (+3) state which is determined by the presence of oxidants and reductants in the medium. Myoglobin (Mb) concentration varies depending on the species, breed, sex, age, type of muscle, exercise, and nutrition. Myoglobin supplies oxygen to the muscle and is responsible for color in meat products, binding oxygen, water, or nitric oxide in the sixth coordination site on the iron molecule. The bright-red color of fresh raw meat surface is due to the great affinity of Mb for oxygen that forms oxymyoglobin; this reaction is rapid and reversible. The interior of meat is purple-red in color due to the pigment called deoxy-Mb that persists as long as reductant compounds are available. This pigment is responsible for the color of vacuum-packed meat. When the reductant concentration is depleted, or the oxygen pressure is low, the heme iron is oxidized to the ferric state, unable to bind oxygen, forming the brown pigment metmyoglobin. In raw fresh meat, these pigments are constantly interconverted (Lawrie 1998; Keeton and Eddy 2004).” (Cheung & Mehta, 2015)

Connective Tissue Proteins
“The main proteins in connective tissues are collagen and elastin. Collagen is the most abundant protein and includes several types of polypeptide chains. Type I collagen is the major component of epimysium and perimysium, while types III, IV, and V collagen are in the endomysium. Collagen fibers are formed by long tropocollagen molecules. Each tropollagen molecule consists of three polypeptide chains twisted together into a coiled triple α helix. The polypeptide chains have the repeating sequence [–glycine–proline–hydroxyproline–glycine–(one of the other amino acids). . .]. Proline and hydroxyproline are approximately 25 % and glycine 33 % of the total amino acid residues. The N-terminals, non-helical regions of the α chains, are forming intermolecular cross-links that are important in collagen resistance in living animals and also in meat toughness. Cross-links are covalent bonds in which lysine and hydrolysine are involved; the enzyme lysyl oxidase catalyzes the oxidative deamination giving aldehyde groups that condense with those from other lysine residues. The cross-links increase with age and augment collagen insolubility and hardness of matured animal meat (Lawrie 1998; Warriss 2000). Under heating (60–65 oC) in moist conditions, collagen swells, its triple helix structure is destroyed at a great extent and transforms into random coils; then, it is called gelatin, becomes soluble, and can retain and immobilize high amounts of water and form gels after rapid cooling to 4 oC (Keeton and Eddy 2004; Belitz et al. 2009).” (Cheung & Mehta, 2015)
“Elastin can be found in lower amounts and is located in capillaries, ligaments, tendons, and nerves. This protein contains about 40 % glycine, 40 % hydrophobic amino acids (18 % valine), and small amounts of proline and hydroxyproline and suffers cross-linking by lysyl oxidase action. Elastin becomes more insoluble with the increase of animal age (Lawrie 1998; Toldrá and Reig 2012).” (Cheung & Mehta, 2015)



Intramuscular Connective Tissue in Muscle Function
Purslow (2020) considered the structure and role of intramuscular connective tissue in muscle function. He found that there is some evidence for myofascial force transmission between fascicles via the perimysium. The variations in the extracellular matrix (ECM) network appears to be linked to the amount of shear displacements between fascicles that must necessarily occur when the whole muscle contracts and changes shape. The two extracellular matrix structures he refers to are the perimysium and endomysium. Together with the epimysium, they form the intramuscular connective tissue (IMCT). Purslow writes that there are large variations in the amount and composition of IMCT between functionally different muscles. Although IMCT acts as a scaffold for muscle fiber development and growth and acts as a carrier for blood vessels and nerves to the muscle cells, the variability in IMCT between different muscles points to a role in the variations in active and passive mechanical properties of muscles. Some traditional measures of the contribution of endomysial IMCT to passive muscle elasticity relied upon tensile measurements on single fiber preparations. These types of measurements may now be thought to be missing the important point that endomysial IMCT networks within a muscle fascicle coordinate forces and displacements between adjacent muscle cells by shear and that active contractile forces can be transmitted by this route (myofascial force transmission). The amount and geometry of the perimysial ECM network separating muscle fascicles varies more between different muscle than does the amount of endomysium.
“It is common for previous literature to describe the endomysium and perimysium as tubes or sheaths that surround each fiber and fascicle, respectively, giving the impression that these “sheaths” individually surround and separate each fiber and fascicle. In reality, the endomysium forms a continuous three-dimensional network throughout the fascicle, provided a connection between adjacent muscle fibers rather than separating them. The perimysium is also a continuous three-dimensional network that runs the length and breadth of the muscle, linking the muscle fascicles that lie in the interstices of this network.” Purslow (2020)
“It is a common assumption that muscle fibers typically run the entire length of a muscle fascicle, inserting onto tendons by myotendinous junctions at both ends. However, numerous studies on a wide variety of species have shown that many muscles have muscle fibers that do not span then entire fascicle, Muscles with non-spanning or intrafascicularly terminating muscle fibers are actually quite common (Gaunt and Gans, 1993; Trotter, 1993; Hijikata and Ishikawa, 1997). Hijikata and Ishikawa (1997) distinguish between non-spanning fibers that terminate on tendinous insertions in some muscles (e.g., mammalian rectus abdominis) and those having short muscle fibers that taper down at each end and terminate within the fascicle, with no connection to the tendons or tendinous insertions (intrafascicularly terminating fibers), the series-fibred muscle. For example, the main locomotory muscles of birds (pectoralis muscles) are series fibers in 63 species studied, from hummingbirds to turkeys (Gaunt and Gans, 1993). In series fibred muscles, connections between fibers via the endomysium are the only possible route for contractile force transmission.” Purslow (2020)
Structure of the Endomysium
“Each muscle fiber (cell) is bounded by its plasmalemma (sarcolemma) and, external to this, a 50 nm thick basement membrane layer comprized of non-fibrous type IV collagen and laminin in a proteoglycan matrix. Lying between the two basement membranes of two adjacent muscle fibers, the fibrous network layer of the endomysium forms a continuum between the two basement membranes. Schmalbruch (1974) estimated that this network layer can be between 0.2 and 1.0 μm in thickness. The fine collagen fibers that make up the bulk of the network layer, together with an amorphous proteoglycan matrix, comprise a planar feltwork of quasi-randomly orientated, wavy fibers (Figure 1E). Transmission electron micrographs of cross sections through the endomysium show that all the collagen fibers run in the plane parallel to the muscle fiber surfaces (Trotter and Purslow, 1992). The preferred orientation of the collagen fibers in the endomysial network changes with muscle sarcomere length, but at all sarcomere lengths the great majority of collagen fibers are still wavy (Purslow and Trotter, 1994) and therefore relatively compliant in tension.” Purslow (2020)
Structure of the Perimysium
“A considerable amount of experience has been accumulated in our laboratory in dissecting out large sheets of perimysium from the bovine semitendinosus muscle for mechanical testing and thermal analysis in a series of publications ranging from 1989 (Lewis and Purslow, 1989) up until the present day (Latorre et al., 2019), which leads this author to the conclusion that perimysium does indeed form a continuous network across the width of a muscle and from origin to insertion of fascicles. It is also clear that the thickness and spatial distribution of perimysium varies greatly between different muscles, as shown in Figure 2 for three bovine muscles (Purslow, 1999). It should also be noted that the resilient protein elastin is present in small amounts in the perimysium of most muscles but that the amount of elastin is increased dramatically in muscles such as bovine latissimus dorsi and semitendinosus (Bendall, 1967), where it is thought to act as an elastic energy store. Rowe (1986) showed that elastin fibers were predominantly associated with the perimysium and epimysium of bovine semitendinosus and longissimus dorsi muscles.” Purslow (2020)
Structure of the Epimysium
“The epimysium is a thick connective tissue layer that is composed of coarse collagen fibers in a proteoglycan matrix. The epimysium surrounds the entire muscle and defines its volume. The arrangement of collagen fibers in the epimysium varies between muscles of different shapes and functions. For instance, the collagen fibers in the relatively thin epimysium of the long strap-like M. sternomandibularis in the cow has two sets of collagen fibers running at ± 55 to the muscle long axis (Purslow, 2010), whereas the collagen fibers in the thicker epimysium of M. semitendinosus in the same animal are close-packed and parallel to the muscle long axis, and merge into the tendon. The thicker epimysium of pennate muscles form a sheet-like aponeurosis that acts as a wide base of muscle attachment (Sakamoto, 1996).” Purslow (2020)
Continuity of IMCT, Tendons and Deep Fascia
“Although the various IMCT structures are often described as sheaths that separate individual fibers (endomysium) fascicles (perimysium) and whole muscles (epimysium), in reality these structures form continuous networks that connect and coordinate the muscle elements within them. The endomysium clearly forms a continuous network structure within a fascicle and perimysium clearly forms another continuous network within the whole muscle As the perimysium approaches the surface of the muscle it merges seamlessly with the epimysium (Turrina et al., 2013). At the ends of the muscle, the epimysium thickens and merges with the tendons (Benjamin, 2009). Tendinous connections from several muscles onto the deep fascia of limb muscles have been observed, and it appears that these connections provide myofascial continuity between the different muscles of the limb (Stecco et al., 2007). It is hypothesized that this continuity of connections between IMCT and fascia coordinate the action of agonistic muscles. Within this hierarchy of connections, the nature of connections between the endomysial and perimysial networks at the surface of muscle fascicles. is less well defined. Rowe (1981) observed an open network of fine wavy collagen fibers joining the thick, dense planar network of collagen fiber bundles in the perimysium to the endomysium of muscle fibers at the surface of a muscle fascicle. Passerieux et al. (2006) similarly reported connections between the perimysium and the endomysium of muscle fibers at the surface of fascicles in bovine flexor carpi radialis muscle that they termed perimysial junction plates (PJPs). These periodic junctions are formed by branching collagen fiber bundles from the perimysium inserting into the surface of the endomysium. Gillies and Lieber (2011) show some evidence of similar connections in their scanning electron micrographs of preparations from mouse extensor digitorum longus muscles. PJPs are staggered at the surface of each muscle fiber and separated by a distance of approximately 300 μm. Transmission electron microscopy and immunohistochemistry studies of PJPs have revealed a concentration of muscle fiber nuclei and mitochondria in the muscle fiber underneath the PJP (Passerieux et al., 2006). This suggests that PJP’s may be a point of transmission of mechanical information and stimuli into the muscle fibers which affects expression in the cell (i.e., points of mechanotransduction). A point of debate is whether these junctions can also function as pathways for transmission of active and passive forces. Passerieux et al. (2006) noted that fracture removed the dense layer of perimysium from the surface of the fascicle, leaving only the perimysial collagen strands attached to PJPs. They argue that this means that these junctions are very strong. However, these connections are only sporadic (more than 100 sarcomeres apart) and mechanical tests (Lewis and Purslow, 1990) showed that the breaking strength of endomysial junctions was considerably below the strength of the perimysial network, indicating that these junctions may not be strong enough to transmit large forces.” Purslow (2020)
Physiological Roles of IMCT
“Intramuscular connective tissue has a wide range of functions. At the most mundane level, it organizes and carries the neurons and capillaries that service each muscle cell. Especially at the level of the perimysium, it provides the location of intramuscular deposits of fat. It patterns muscle development and innervation, as proliferation and growth of muscle cells is stimulated and guided by cell–matrix interactions. These roles have been discussed previously (Purslow, 2002). This review focuses on the current knowledge of the mechanical roles of IMCT in relation to the transmission of contractile force, passive tension in stretched muscle, and the adaptation of muscle due to mechanotransduction.” Purslow (2020)
“Hill (1949) stated that the mechanical properties of skeletal muscle could be described by a contractile element (CE), an elastic element in series with this (SE) and another Eleatic component in parallel to the CE and SE. He was at pains to point out that this was a mechanical description only, and that it was not necessary to identify the structures responsible for the SE and PE response. By “elastic” he meant that these elements would return to their original dimensions after loading, and so act as spring-like stores of strain energy. He noted that the stress-strain behavior of his hypothetical SE and PE elements would be non-linear, in that their stiffness would increase with strain. This “Hill-type three element model” conditioned many discussions of the mechanical behavior of muscle subsequently. This model essentially focuses on the contribution of each “element” in tension, a constraint that is discussed below.” Purslow (2020)
Experimental Data from Purslow (2020)
“Two papers published in the mid 1980’s characterize two very different streams of thought about the contribution of IMCT to the mechanical functioning of muscle. Magid and Law (1985) measured the passive load-extension behavior of single fibers from frog muscle with and without surrounding endomysium and concluded that this IMCT structure contributed very little to the passive tension of muscle. This finding generated a focus on titin as the principal intracellular structure responsible for passive elasticity in the tissue. Looking at Figure 1E, it is clear that dissection of a single muscle fiber with some surrounding endomysium must involve the disruption of the continuous network structure of the endomysium, a process that is much easier to achieve without damaging the muscle fiber in frog muscle than in mammalian species, hence frog muscle being the material of choice for single fiber physiology studies. It is more difficult, but possible, to isolate viable single fibers from mouse extensor digitorum longus muscle and measure their properties (Lännergren and Westerblad, 1987, 1991; Westerblad et al., 1993). Measurements of the passive elasticity of muscle by tensile tests on single fibers have also been used to look at changes in IMCT in diseased human muscle (Mathewson et al., 2014) or changes due to unloading on rat soleus fibers (Toursel et al., 2002). Meyer and Lieber (2018) made a direct comparison of the passive elasticity of mouse versus frog single fibers using the same apparatus and protocol, and showed that endomysium had a greater contribution to passive elasticity in the mouse muscle fibers than those from the frog. By testing the tensile properties of whatever remnant of the endomysial network that clings to the surface of an isolated single fiber, these investigations unequivocally demonstrate the non-linear tensile properties of the endomysium. The thinking behind this line of investigation is very much in accord with the three-element Hill-type model of muscle mechanics that distinguishes a contractile element, a series-elastic element and a parallel elastic element. By equating the endomysium with the parallel elastic element, this type of analysis forces our thinking into consideration of the tensile properties of the endomysium. Purslow and Trotter (1994) studied changes in the orientation and waviness of collagen fibers in the endomysial network layer with muscle sarcomere length and found that endomysium is non-linearly elastic but extremely compliant in tension over the physiological range of sarcomere lengths. A similar investigation with perimysium also showed that this network of crimped collagen bundles is very compliant in tension over the range of physiologically relevant sarcomere lengths (Purslow, 1989). By constraining thought to tensile properties of these planar fibrous networks of wavy collagen fibers or fiber bundles, it was concluded that IMCT structures are too compliant in tension to efficiently contribute to the transmission of contractile force at the sarcomere lengths where muscle generates most force. However, as noted above, this conclusion is simply based on the assumption that these ECM structures are working in tension. As discussed below, that is now thought to be incorrect, and that their through-plane shear properties are more important. Using finite element models based upon the Hill-type three-element model, Marcucci et al. (2019) have recently suggested that the contribution to the parallel elastic component in passive muscle elasticity may nevertheless be substantial.
The second stream of thought about the functioning of connective tissue within muscle was generated by the observation by Street (1983) that short segments of myofibrils from adjacent fibers adhering to the endomysium of a single fiber dissected from frog semitendinosus muscle changed length when the intact fiber was stretched. This gave birth to the idea that forces are transferred laterally between adjacent fibers by shear through the endomysium. This mechanism explains why length changes in non-activated muscle fibers follow the length changes of actively contracting neighboring fibers when only a subset of muscle motor units are activated in sub-maximal contraction.
A general recognition that force transmission can readily occur between adjacent muscle fibers has been followed by evidence that myofascial force transmission can occur between fascicles even between adjacent muscles, as summarized by Huijing (2009); Maas and Sandercock (2010), and Maas (2019). While there is some dispute that epimysial force transfer between individual muscles is significant (Diong et al., 2019), the general idea of lateral force transmission between adjacent fibers within a muscle fascicle is less controversial. However, this concept requires a change of mental picture about the functional properties of IMCT is two ways. Firstly, although it is possible to discern the tensile properties of endomysium by comparing the tensile properties of skinned single muscle fibers to muscle fibers with endomysium, or small groups of fibers with a part of intact endomysial network between them, the relevance of this needs to be rethought. If a prime function of the endomysium is to coordinate strains between adjacent muscle fibers and keep sarcomeres in register with each other by transmission of forces by shear, does measurement of tensile properties really help to understand these important shear properties? Secondly, from a materials science or biophysical view of endomysium and perimysium, it is natural to focus on the tensile, in-plane properties of a planar fibrous network and the non-linear behavior that these exhibit due to strain induced reorientation and de-crimping. This was the approach originally applied to analysis of the perimysium (Purslow, 1989) and to the endomysium (Purslow and Trotter, 1994), and in both cases the result was only to highlight the worryingly high compliance of these networks in tension. It should be remembered that the endomysium studied by Purslow and Trotter (1994) was from a very obviously series-fibred muscle (bovine sternomandibularis muscle) where the none of the intrafascicularly terminating, short muscle fibers run the length of a fascicle and the great majority have no myotendinous attachment, so that transmission of contractile force via the endomysium is the only option. We should also note that the endomysial connections between intrafascicularly terminating fibers in series-fibred muscles are essentially acting as part of the series elastic component in Hill’s three-element model; efficient transfer of force from the contractile element out to tendons and eventually bones requires a series-elastic “link” that (a) does not dissipate energy (which would waste the energy of contraction) but stores it elastically, and (b) is relatively stiff, as a very stretchy or compliant linkage would not efficiently translate muscle contractions into movement of the bones. Analysis of tensile properties of these planar collagenous networks continues to be a common mindset (e.g., Bleiler et al., 2019). The highly compliant tensile properties of endomysium provide little resistance to the longitudinal and circumferential dimensional changes in working muscle fibers; the endomysium easily allows and follows changes in fiber geometry as muscle contracts and is passively lengthened. This, however, is not inconsistent with providing a reasonably efficient transmission of force by translaminar shear (shear through its thickness).
Tensile tests on small sheets of perimysium isolated by careful dissection from muscle are possible and show the obvious non-linear stress-strain behavior expected of a compliant network that suffers reorientation at finite strains and a straightening of initially wavy or crimped collagen fiber bundles. An example if given in Figure 3. Tensile tests on isolated perimysium from large bovine muscles have continued to be performed only because of the relevance of these properties to the textural properties of muscle eaten as meat (e.g., Latorre et al., 2019), but of course do not shed light on the functioning of this IMCT structure in vivo, except to reinforce the point that perimysium, like endomysium, is easily deformed in tension at resting muscle lengths.
It is clear that the majority of muscles undergo shape changes as they contract (Dick and Wakeling, 2017, 2018; Roberts et al., 2019); fusiform muscles bulge in mid-section as they contact, as do fan-shape muscle such as the pectoralis and all unipennate, bipennate and multipennate muscles. This sounds like a trivial observation, but consideration of how a fibrous composite tissue can change shape reveals that, in order to do so, some elements in the tissue as a whole must be allowed to shear past neighboring elements. The question is; which elements, at what scale of structure? If the endomysium is tightly coordinating forces and displacements between adjacent muscle fibers in a fascicle, then the likelihood is that shear displacements could be accommodated between fascicles. In a crude experiment, Purslow (2002) demonstrated that shape changes caused by manipulation bovine semitendinosus muscle in rigor produced slippage between fascicles, but not within fascicles. Schmalbruch (1985) also discusses this mechanism. The shear strains within different muscles are substantial and vary between diverse muscles (Mutch, 2015).
It has been postulated that variations in the size and shape of fascicles, and therefore in the spatial distribution of perimysium, was related to variations in the shear strains that need to be accommodated in differently shaped muscles as they contract (Purslow, 2002, 2010). This idea has since been supported by computational models (see below) and argues that shear stains in the perimysium must be larger than shear strains though the endomysium between muscle fibers in a fascicle. This is in contrast to the interpretation of those researchers (e.g., Huijing, 2009; Maas, 2019) who stress the importance of lateral force transmission between fascicles and between entire muscles (epifascial force transmission) as an important physiological function, as a perimysium easily deformed in shear would not be an efficient means to transmit contractile force laterally between fascicles. Purslow (2020)
I have to go no further in trying to understand the loss of structure in meat that has been comminuted beyond a certain micron size. For more information on this fascinating discussion, I post Purslow (2020)’s entire article below under “Further Reading.“
Post Mortem Pressure Treatment of Meat Intramuscular Connective Tissue and Observed Structure
If pressure by itself negatively impacts the structure of beef is uncertain. Voutila (2009) concludes that “the reports about effects of pressure treatments on meat tenderness seem contradictory: Beef hardness (measured with Texture Profile Analysis) has been successfully reduced with high pressure treatment by Ma and Ledward (2004), but according to Macfarlane et al. (1981) pressure treatment did not lower the Warner Bratzler shear force values. Ma and Ledward (2004) suggested that the high pressure treatment accelerated the proteolysis post mortem in meat but did not affect the structure of meat itself. The Tp of IMCT or solubility of intramuscular collagen does not seem to be affected by pressure treatments (Suzuki et al., 1993; Ma & Ledward, 2004). (Voutila, 2009)
Enzymes
“Many enzymes can be found in the muscle and most of them are peptidases: calpains, cathepsins, proteasome, tri- and dipeptidylpeptidases, amino- and carboxypeptidases, and dipeptidases. Other enzymes are lipases, glycohydrolase, nucleotidases, etc. Peptidases are important in meat tenderness and texture and flavor development in meat products and can be divided into two groups: endopeptidases, endoproteases, or proteinases (calpains, cathepsins, and proteasome), when they hydrolyze internal peptide bonds, and exopeptidases, when they hydrolyze external peptide bonds at the carboxy or the amino termini of the protein/peptide chain.” (Cheung & Mehta, 2015)
“Calpains are a group of Ca2+-dependent cysteine endopeptidases (110 KDa) which show maximal activity at neutral pH values (pH 7.5) and become ineffective activity at pH 5.5. The most important isoforms are calpain 1 or μ-calpain and calpain 2 or m-calpain. Calpain 1 requires micromolar calcium concentrations (10–50 μM) for activity and has low stability in postmortem muscle. Calpain 2 needs millimolar Ca2+ concentrations (0.3–1.0 mM) for full activity and seems to be stable for a few weeks postmortem, up to 56 days. Their activity is regulated by an endogenous inhibitor, calpastatin, that disappears few days after slaughter by autolysis. Another isoform, p94/calpain 3 isoform, is active even without calcium ions in the medium; its role in the muscle and in postmortem aging is not well known. Calpains can degrade a wide variety of myofibrillar proteins responsible for the fiber structure (titin, troponins T and I, tropomyosin, C protein, filamin, desmin, and vinculin), but they are inactive against myosin and actin. These proteases are considered as the predominant enzymes that cause postmortem proteolysis and meat tenderization. Calpains are particularly susceptible to inactivation by oxidation, although this might not completely inhibit proteolysis.” (Cheung & Mehta, 2015)
Cathepsins are a group of over 15 lysosomal small proteinases (20–40 KDa) that are distinguished by their active sites and substrate specificity. Main cathepsins are cathepsins B and L (cysteine proteinases, very active at pH 6.0), cathepsin H (endo and exopeptidase, optimal pH at 6.8), and cathepsin D (aspartate proteinase, optimal activity at pH 3.0–5.0). Sometimes, cathepsins are not considered as important in meat tenderization because of their location into lysosomes of muscles cells; however, lysosome membranes can rupture during postmortem period due to the decreasing temperature and pH, and cathepsins B, D, H, and L have activity during postmortem aging and also during meat product manufacture. These proteinases can degrade different myofibrillar proteins (myosin, actin, tropomyosin, troponins, titin, and α-actinin), some collagen cross-links, and mucopolysaccharides of the connective tissue.” (Cheung & Mehta, 2015)
“Proteasome is a large multicatalytic protease with chymotrypsin- and trypsinlike activities and peptidyl-glutamyl hydrolyzing activities at neutral pH values. It degrades myofibrils affecting M- and Z-lines and could play a role on tenderness in some muscles.” (Cheung & Mehta, 2015)
“There are several types of exopeptidases, located in the lysosomes or in the cytosol, that release small peptides and amino acids important for meat taste. Tripeptidylpeptidases are enzymes that hydrolyze tripeptides from the amino termini of peptides; dipeptidylpeptidases are able to hydrolyze the amino termini of dipeptides. Aminopeptidases are neutral or basic metalloproteases of high molecular mass that release amino acids from the amino termini of peptides, while carboxypeptidases, located in lysosomes, generate amino acids from the carboxy termini of peptides and proteins (Warriss 2000; Huff Lonergan et al. 2010; Toldrá 2012).” (Cheung & Mehta, 2015)
“Lipolytic enzymes degrade lipids with different modes of action and substrates. Lipases are situated in lysosomes and in cytosol and are located in the skeletal muscle and in the adipose tissue. In the skeletal muscle, lysosomal acid lipase and acid phospholipase release long-chain free fatty acids from triacylglycerols (at positions 1 or 3) and phospholipids (at position 1), respectively, and are active at acid pH (4.5–5.5). Phospholipase A and lysophospholipase are basic lipases that release fatty acids from phospholipids at positions 1 and 2, respectively, and are more active in oxidative muscles. Acid and neutral esterases hydrolyze short-chain fatty acids from tri-, di-, and monoacylglycerols.” (Cheung & Mehta, 2015)
“In adipose tissue, the most important lipase is hormone-sensitive lipase that hydrolyzes long chain diacylglycerols and triacylglycerols, with specificity for positions 1 and 3. Other adipose tissue lipases are lipoprotein lipase and monoacylglycerol lipase; this last enzyme produces glycerol and free fatty acids as end products (Toldrá 2012).” (Cheung & Mehta, 2015)
“Meat also contains lipoxygenase, an enzyme that catalyzes the incorporation of molecular oxygen into PUFA, mainly arachidonic acid; the final product of the reaction is a conjugated hydroperoxide. It remains active and stable during frozen storage of meat and develops rancidity in frozen chicken (Toldrá 2012).” (Cheung & Mehta, 2015)

Nonprotein Nitrogen Compounds and Other Minor Constituents
“Free amino acids are present in muscle (0.1–0.3 %) in part due to the action of muscle aminopeptidases and are more abundant in oxidative than in glycolytic muscles. The most predominant amino acids are taurine (0.02–0.1 %), alanine, and glutamic acid (0.01–0.05 %). Their content increases during postmortem storage of meat (Belitz et al. 2009; Toldrá and Reig 2012).” (Cheung & Mehta, 2015)
“Meat contains in variable amounts three natural dipeptides that develop physiological functions in the muscle (buffers that maintain the muscles in the physiological range, antioxidants, neurotransmitters, etc.): carnosine (β-alanyl-Lhistidine), anserine (β-alanyl-L-1-methylhistidine), and balenine (β-alanyl-L-3-methylhistidine). Carnosine and anserine are present in all species (0.01–0.3 %); beef and pork have a higher content of carnosine than anserine, lamb has similar concentrations of both, and poultry is rich in anserine. Balenine is more abundant in whales’ muscles, shows minor amounts in pork muscle, and is very low in other animals. In whales this may be an adaptation to the anaerobic metabolism and the improvement of lactic acid concentration during diving (Warriss 2000; Toldrá and Reig 2012).” (Cheung & Mehta, 2015)
“Minor constituents of meat are amines, guanidine compounds (creatine and creatinine), quaternary ammonium compounds (choline and carnitine), and nucleotides (Belitz et al. 2009).” (Cheung & Mehta, 2015)
Lipids
“Lipids in meat animals are commonly classified as depot fats and intramuscular lipids. Depot fat is localized as subcutaneous fat, between muscles as intermuscular fat and in the body cavity around kidneys, heart, and pelvic regions (Rhee 1992). Most of the fat in the body are localized in these deposits. The proportion of the total body fat in each fat depot varies between species (Warriss 2000). Triglycerides (triacylglycerols) are the main lipid component (>90 %) of these adipose tissues (Wood et al. 2008). Low quantities of other components such as diglycerides, monoglycerides, free fatty acids, fat-soluble vitamins, and cholesterol esters can be also found in depot fat.” (Cheung & Mehta, 2015)
“Intramuscular lipids represent a low percentage of total lipids of the body. For example, in mature pigs, about 15 % of the extractable lipids are in intramuscular fat. Muscles contain around 5 % lipids. However, this content is very variable, ranging from 1 % to 15 % (Kauffman 2012). Lipids are necessary to increase flavor, juiciness, tenderness, and visual characteristics of meat. Meat with very low levels of lipids has poor organoleptic evaluation by the consumers. However, high contents of lipids in meat that are visible by the consumers are not appreciated because the meat fat is related to the incidence of some diseases cardiovascular disease, obesity, and cancer).” (Cheung & Mehta, 2015)
“Intramuscular lipids are composed of deposit and structural lipids. Although some lipids are found inside of the muscle cells, deposit lipids in muscles are mainly localized in intramuscular adipocytes (Cobos et al. 1994). This depot is commonly called marbling. As in other depot fats, triglycerides are the main component and there are small amounts of other substances such as diglycerides, monoglycerides, free fatty acids, fat-soluble vitamins, and cholesterol esters.” (Cheung & Mehta, 2015)
“Structural lipids are in membranes of muscle and intramuscular adipocytes, being phospholipids and cholesterol the main constituents. The amount of total phospholipids in the muscle stays relatively unchanged (3.5–6.0 mg/g); however, the proportion of phospholipids in relation to total lipids can change due to the increase in the amount of triglycerides. The relative amount of phospholipids can range from 10 % to 50 %. Depending on the type of meat, age of animals, and other potential factors such as diet, there are large variations in total lipids and phospholipids. The most common phospholipids in muscle tissue are phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and sphingomyelin with around 40 %, 15 %, 10 %, and 5 % of total phospholipids, respectively (Willian 2013). Lower proportion of other phospholipids as plasmalogens and other polar lipids as cerebrosides can be found in muscle membranes.” (Cheung & Mehta, 2015)
“Cholesterol content of meat is around 50–70 mg/100 g and this amount is partly independent of the fat content (Leth and Ertbjerg 2004). Cholesterol content of adipose tissues is around 115 mg/ 100 g tissue (Smith et al. 2004). Cholesterol in meat exists as free cholesterol and as cholesterol ester. Free cholesterol is associated primarily with cellular and subcellular membranes of muscle and intramuscular adipocytes. Cholesterol ester is located with the triglycerides in adipose tissue. Muscle fibers, which are rich in membranes and poor in lipids, have around 75 % of their total cholesterol associated with membranes and the other 25 % associated with triglycerides. Intramuscular adipocytes, which are rich in lipids and poor in membrane content, have small proportion (around 25 %) of cholesterol associated with membranes (Smith et al. 2004).” (Cheung & Mehta, 2015)
“Cholesterol content of meat is around 50–70 mg/100 g and this amount is partly independent of the fat content (Leth and Ertbjerg 2004). Cholesterol content of adipose tissues is around 115 mg/ 100 g tissue (Smith et al. 2004). Cholesterol in meat exists as free cholesterol and as cholesterol ester. Free cholesterol is associated primarily with cellular and subcellular membranes of muscle and intramuscular adipocytes. Cholesterol ester is located with the triglycerides in adipose tissue. Muscle fibers, which are rich in membranes and poor in lipids, have around 75 % of their total cholesterol associated with membranes and the other 25 % associated with triglycerides. Intramuscular adipocytes, which are rich in lipids and poor in membrane content, have small proportion (around 25 %) of cholesterol associated with membranes (Smith et al. 2004).” (Cheung & Mehta, 2015)
“The fatty acids in meat are mainly found in triglycerides and phospholipids. In depot fats, the fatty acids are found mainly in triglycerides. In muscles, the contribution in the total fatty acids of these compounds depends on the total fat content. The phospholipids contribute between 10 % and 40 % of the total fatty acids in the muscle (Wood et al. 2008).” (Cheung & Mehta, 2015)
“Most fatty acids in meat contain between 14 and 20 carbon atoms in the molecule. The total content of saturated fatty acids (SFA) is 30–50 %. The main SFA are palmitic acid (C-16:0), stearic acid (C-18:0), and myristic acid (C-14:0). Palmitic acid is the most abundant (20–25 %) followed by stearic acid (5–20 %).” (Cheung & Mehta, 2015)
“About 35–50 % of fatty acids are monounsaturated, oleic acid (C-18:1) (30–45%) being the main monounsaturated fatty acid (MUFA) followed by palmitoleic acid (C-16:1) (2–5 %).” (Cheung & Mehta, 2015)
“A smaller proportion of fatty acids in meat are polyunsaturated (2–30 %). Polyunsaturated fatty acids (PUFA) are of the n-6 and n-3 family. The most important n-6 fatty acids in meat are linoleic acid (C-18:2 n-6) and arachidonic acid (C-20:4 n-6); the n-3 fatty acid present in the largest quantity is α-linolenic
acid (C-18:3 n-3). Double bonds in MUFA and PUFA are mainly of the cys-type. Trans fatty acids, fatty acids with conjugated double bonds [such as conjugated linoleic acids (CLA)], fatty acids with an odd number of carbon atoms, and fatty acids with branched chains are present in higher proportion in ruminant meat than in nonruminant meats. Ruminant meat (beef, lamb, goat) has more complex fatty acid composition than those of nonruminants due to the activity of microorganisms in rumen. These fatty acids are absorbed in the small intestine and incorporated into meat (Wood et al. 2008). The trans fatty acids C-18:1 are present at levels of 2.8–4.7 % in meat from beef and lamb, respectively; however, these fatty acids are not detected in pork meat (Wood et al. 2008). The levels of CLA in meat from lamb (4.3–19.0 mg/g lipid) and beef (1.2–10.0 mg/g lipid) are higher than in pork and chicken (lower than 1 m/g lipid); it is very interesting due to the positive effects on cancer, cardiovascular disease, diabetes, body composition, immune system and bone health of this fatty acid (Schmid et al. 2006). CLA describes a mixture of positional and geometric isomers of linoleic acid; among them, the most studied isomers are cis 9, trans 11 and trans 10, cis 12-CLA.” (Cheung & Mehta, 2015)
Carbohydrates
“Carbohydrates are present in a relatively small concentration in living muscle tissue, ranged from 0.5 % to 1.5 %. The main carbohydrate is glycogen, a branched polysaccharide composed of α-D-glucose units (up to 50,000) linked by α-1,6 glucosidic and α-1,4 glucosidic bonds, which in living animal functions as an energy store supplying energy for muscle contraction through aerobic glycolysis. After death, no oxygen is available from blood, aerobic pathways stop, and this causes, for a short period of time, a conversion to anaerobic glycolysis, in which glucose is converted into lactate. At 24 h postmortem, glycogen concentration drops to less than 1 %. Other carbohydrates include glucose, other mono- and disaccharides (0.1–0.15 %), and intermediates of glycogen metabolism (Warriss 2000; Keeton and Eddy 2004).” (Cheung & Mehta, 2015)
Vitamins
“Meat and meat products are good sources of most of the water-soluble vitamins, mainly thiamin (vitamin B1), riboflavin (vitamin B2), niacin, and vitamins B6 and B12. Their concentrations range from a few micrograms (vitamin B12, 0.31–3.1 μg) to several milligrams (niacin, 3.6–12.6 mg) per 100 g. Liver is rich in folate. Red meats, such as beef and lamb, are specially good sources of vitamin B12. Pork meat and products are one of the best sources of thiamin (0.9–1.2 mg/100 g). The content of vitamin C in meat is variable (0–2.3 mg/100 g); meat is not an important source of this vitamin.” (Cheung & Mehta, 2015)
“Regarding fat-soluble vitamins, vitamin A is detected in small amounts in meat and meat products (0–40 μg/100 g) with the highest values in meat with high lipid content and in larger amounts in the liver (15,000 μg/100 g), mainly as all-transretinol, and smaller amounts of 13-cis-retinol. Vitamin D and its metabolite 25-hydroxy vitamin D are also present in some meat products, but at very low levels [0.03–0.60 μg/100 g vitamin D3 (cholecalciferol) and 0.4–0.20 μg/100 g 25-hydroxy vitamin D]. Meat is not an important source for the other fat-soluble vitamins, that is, vitamins E and K, that are present at low levels (0.16–0.69 mg and 0.0–6.8 μg per 100 g, respectively) (Leth and Ertbjerg 2004; Lofgren 2005; USDA
2014).” (Cheung & Mehta, 2015)

Minerals
“Meat is a very rich source of some minerals: phosphorus, potassium, magnesium, iron, copper, zinc, and selenium. Iron content is very important as nutrient (mainly in red meat) because it is present in the high bioavailable heme form (Lofgren 2005). Other minerals (calcium and sodium) are present at low levels in meat. Mineral content in meat of various species is shown in Table 2.” (Cheung & Mehta, 2015)
Water
“Water constitutes 75 % of lean meat on average. In postmortem muscle, water is the major component of sarcoplasm of muscle cell and surrounds the myofibrillar proteins. There are three types of water in meat: bound, immobilized, and free. Bound water (about 4–5 % of water in muscle, 0.3–0.5 % water/g protein) is held tightly by myofibrillar protein charges and it is also referred as constitutional water. Several amino acids can attract and bind water in myofibrillar proteins: glutamic acid and lysine due to charged side groups and glutamine and tyrosine that contain nitrogen and oxygen atoms in side groups with sufficient polarity to attach water. Bound water cannot move among water compartments, remains unfrozen at -40 oC, and can only be eliminated by severe drying.” (Cheung & Mehta, 2015)
“Immobilized or entrapped water is the largest proportion of water bound in meat; it is retained in the muscle ultrastructure by either steric effects or by attraction to bound water, but not directly bonded to the myofibrillar proteins. It can be removed by conventional heating and converted into ice during freezing.” (Cheung & Mehta, 2015)
“Free water is held in the meat by weak capillary force and flows from meat unimpeded. It is not readily observed in pre-rigor meat and appears when the entrapped water moves during rigor and post-rigor changes.” (Cheung & Mehta, 2015)
“Water holding capacity (WHC) is defined as the ability of meat to retain its inherent water during force application and/or processing (grinding, curing, thermal processing, etc.) and also the water added during meat product manufacturing. Myofibrils play a predominant role in the water holding capacity of meat. Losses of water can occur via evaporation, gravitational drip, thawing, or cooking. Water content is important because it affects weight, consumer acceptability, and functional properties of fresh meat and meat products. Low water holding capacity and, consequently, excessive moisture loss result in considerable economic and quality losses.” (Cheung & Mehta, 2015)
“Several factors contribute to WHC: antemortem factors, such as genetics, animal production practices, carcass chilling, stress, and postmortem items, that include factors related to the transformation of muscle into meat (mainly pH), heating, and addition of other components (sodium chloride, phosphates, etc.).” (Cheung & Mehta, 2015)
“During postmortem changes, the accumulation of lactic acid, due to anaerobic metabolism, is responsible for the decline of pH muscle from about 7 to values of 5.4–5.7 at 24 h after slaughter. When muscle pH falls to values near the isoelectric point (pI) of the main myofibrillar proteins (about 5.0–5.3), the charged groups of the proteins (virtually equal number of positive and negative charges) are attracted to each other, reducing the interaction with water and the spaces between myofilaments. In this situation, WHC reaches minimum values. WHC increases with the addition of acids or alkalis due to the increment of negative and positive charges of proteins, respectively, that enhance repulsion.” (Cheung & Mehta, 2015)
“During postmortem aging, WHC of meat increases without substantial changes in pH value due to the disorganization of myofibrillar structure. Poor antemortem handling can produce meat of poor quality and altered WHC. Stress preslaughter and stress-susceptible genotypes may alter postmortem changes producing pale, soft, and exudative (PSE), with low WHC, and dry, firm, dark (DFD) meats, with very high WHC, two of the major quality problems for meat industry. Sodium chloride is usually added to meat products and, among other effects, alters the water holding capacity of meat. Salt addition modifies the electric charges of myofibrillar protein groups. Chloride anions bind firmly to positively charged protein groups, while sodium cations are weakly bound to the negatively charged groups. At pH values above the isoelectric point, the binding of chloride ions to proteins increases its net negative charge. This displaces the pI toward a lower pH, produces an increase of repulsion between myofibrillar proteins, and opens the structure, resulting in enhanced water retention of meat. WHC of connective tissue proteins is presumably enhanced by ions too. Conversely, at pH values below pI, the positive charges of protein groups are neutralized by chloride ions, reducing net positive charge and WHC.” (Cheung & Mehta, 2015)
“WHC improves with increasing amounts of added salt from 1.8 % to concentrations higher than 1 M. Maximum swelling of meat proteins is reached at salt concentrations between 0.85 (about 5 %) and 1 M (about 6 %). The water uptake may be caused by the expansion of the grid of myofibrillar filaments as a consequence of the increasing repulsion of negatively charged groups and also by the disruption of forces that determine the arrangement of filaments at the Z- and M-lines and between the myosin heads and actin filaments. Salt concentrations higher than 6 % reduce the swelling effect. This apparent dehydrating effect has been attributed to the precipitation of myosin that would reverse its depolymerization and cause shrinkage. Salt-induced swelling and water expulsion also depend on the postmortem status of the meat as well as the type of muscle and fibers studied and the influence of physical phenomena that depends on the type of salting process applied to the meat (Lawrie 1998; Devine and Chrystall 2000; Warriss 2000; Keeton and Eddy 2004; Apple and Yancey 2013).” (Cheung & Mehta, 2015)
Factors That Influence Meat Composition
Species
“The chemical composition of meat from different species is similar in the percentages of nitrogenous compounds (21–22 %) and ash (1.0–1.1 %), but some differences can be observed in relation to fat content and amounts of cholesterol and some vitamins and minerals (Table 2). Other animals such as duck and rabbit deposit little fat in muscles (Kauffman 2012). Rabbit meat is characterized by a lower content of sodium (37–47 mg/100 g edible fraction) and cholesterol than other meats such as pork, beef, and chicken (Dalle Zotte and Szendro 2011).” (Cheung & Mehta, 2015)

“Important differences between species are related to the fatty acid composition (Table 3). In relation to the meats from main animal species (beef, lamb, pork, and chicken), ruminant meats (beef and lamb) have higher proportion of SFA and lower content of PUFA than pork and chicken. Ruminant meat is more saturated due to the hydrogenation of unsaturated fatty acids by microorganisms in the rumen. Rabbit meat shows higher content of PUFA (32.5 %) than beef, pork, and chicken; the proportion of linoleic acid in rabbit meat is 22 % and the content of α-linolenic acid is 3.3 % (Dalle Zotte and Scendro 2011).” (Cheung & Mehta, 2015)
“Nowadays, there is a growing interest in meat for alternative species (horse, ostrich, game meat, etc.). These meats have low fat and cholesterol contents and high concentration of iron and PUFA (specially the n-3 fatty acids) (Polawska et al. 2013).” (Cheung & Mehta, 2015)
“Myoglobin concentration in meat governs its color and is influenced by species. Beef and lamb (“red meats”) contain substantially more myoglobin than pork and poultry meat (“white meats”) (James and James 2010).” (Cheung & Mehta, 2015)
Other Factors
“Many factors influence the meat composition, being lipids the most variable component. As we described before, the lipid content of the main component of the meat, the muscle, may range from 1 % to 15 %. This variation is due to many factors such as type of muscles, breed, age (stage of growth), sex, physical exercise, and nutrition. The fatty acid composition of meat is also influenced by these factors. Besides, there are differences in the fatty acid composition between muscles and depot fats. Intramuscular lipids have a higher proportion of PUFA than depot fats. This difference is due to the higher proportion of phospholipids in muscle tissue than in depot fats and phospholipids have a higher concentration of PUFA than triglycerides (Rhee 1992).”(Cheung & Mehta, 2015)
“Muscles in the carcass differ in fiber type and in nature and concentration of the connective tissue. These differences influence the chemical composition of the muscles. Myoglobin concentration is higher in red fibers (Kauffman 2012). The fatty acid composition of muscles is also affected by the type of fibers. Red oxidative fibers contain more mitochondria and a higher proportion of phospholipids than white fibers and as a result have a higher proportion of PUFA (Wood et al. 2008). The nature of the connective tissue matrix also affects the accumulation of fat in the muscles. Loosely arranged muscles that have parallel connective tissue strands contain more fat than tightly compacted muscles. The latter’s connective tissue strands are thicker and more tight structures, this prevents excess lipid accumulation. Anatomical location of muscles is also important because some of them have higher contents of tendon and epimysial sheaths of connective tissue. Due to this, differences in the amounts of stroma proteins comparing to myofibrillar,
sarcoplasmic, and granular proteins can be found (Kauffman 2012).” (Cheung & Mehta, 2015)
“Breed types can influence lipid content and fatty acid composition. Some breeds of pigs, cattle, and sheep contain more intramuscular fat for a given degree of body fatness and age. Duroc is an example of breed that accumulates more intramuscular fat in pigs. In bovine species, Angus deposits more intramuscular fat at a given physiological state of maturity than Hereford and Charolais (Kauffman 2012). Effects of breeds and genetic lines on muscle and adipose tissue fatty acid composition have also been reported for ruminants (beef and lamb) and monogastrics (pigs) (Wood et al. 2008).” (Cheung & Mehta, 2015)
“Stage of growth is another important factor: in young animals, the accumulation of proteins is higher; when the muscles stop growing, intramuscular lipids may accumulate, decreasing the proportion of other components. Muscles that mature earlier have the structural potential for accumulating more lipids at a given age than muscles that mature a later stage (Kauffman 2012). Meat fatty acid composition can also be influenced by changes in age (Rhee 1992). In relation to meat proteins, older animals show higher proportion of heat-stable or heat-insoluble collagen crosslinks, and this makes their meat tougher than that of younger animals (Warriss 2000).” (Cheung & Mehta, 2015)
“Exercise simulates fiber hypertrophy and also stimulates mobilization of lipids and decreases of the lipid content in muscles. There is little evidence suggesting changes in other chemical components (Kauffman 2012).” (Cheung & Mehta, 2015)
“The chemical and fatty acid composition can be influenced by sex. The testosterone regulates lipid deposition in muscles. The males have less intramuscular fat than females. Male castrated animals contain more intramuscular fat than non-castrated animals (Kauffman 2012). Differences in fatty acid composition have also been reported between males and females and between castrated and non-castrated males (Rhee 1992).” (Cheung & Mehta, 2015)
“Although several factors can influence chemical composition of meat, diet is the most important factor that can modify chemical composition, specially lipid and fatty acid composition. Diets with high caloric content increase lipid accumulation; in submaintenance diets, lipids are mobilized from muscles. In relation to cholesterol and fatty acids, the effect of the modification of the diet is different. It is difficult to modify cholesterol content by dietary manipulation, except when increasing the amount of intramuscular lipids which will cause small increases in cholesterol concentration (Smith et al. 2004); however, the fatty acid composition of meat is very influenced by animal diet. Fat from the diet constitutes the most important factor that modifies the fatty acid composition of meat lipids (Cobos et al. 1994). The consumption of meat fat is related to increased incidence of cardiovascular disease due to their levels of saturated fat. The objective of the meat industry is the reduction of the levels of palmitic acid and the increase of the proportion of MUFA and PUFA, mainly n-3 fatty acids. Modifying the animal diet is possible to obtain healthier balance of fatty acids to the consumer.”(Cheung & Mehta, 2015)
“The fatty acid composition of meat is sensitive to dietary manipulation, specially in monogastric animals (pigs, poultry and rabbits), since fatty acids are deposited unchanged by digestion. Fatty acid composition of ruminant meat is less influenced by dietary lipid composition. In ruminants, the microorganisms of the rumen modify the fatty acid composition of the diet because they convert unsaturated fatty acids from diet into SFA (Rhee 1992; Cobos et al. 1994; Wood et al. 2008).” (Cheung & Mehta, 2015)
“Diets supplemented with a source of n-6 (such as sunflower oil or soybean oil) or n-3 fatty acids (such as linseed or fish oil) lead to an increased n-6 or n-3 fatty acid content in meat, respectively. These fatty acids are entirely derived from the diet. These results are stronger in monogastrics than in ruminants. However, the PUFA content of ruminant meat can also be increased, to a certain extent, by feeding diets supplemented with polyunsaturated fats. Despite the hydrogenating effect of the conditions of the rumen on dietary PUFA, small but significant amounts enter the duodenum to be absorbed into the blood and delivered to the tissues as in monogastrics (Wood et al. 2008). In ruminants, diets with high levels of PUFA also increase total CLA. In monogastric animals, the only way to increase CLA in meat is the supplementation with CLA or its precursor trans-vaccenic acid in the diet (Schmid et al. 2006). The content of MUFA in meats is increased by incorporation of oils rich in MUFA (such as canola or high-oleic variety of sunflower) into the diets of animals (Rhee 1992).” (Cheung & Mehta, 2015)
“Changes in the fatty acid composition of meat also have effects on meat quality, for example, fat tissue firmness, color, shelf life, and flavor (Wood et al. 2008). The main problem associated to increased PUFA in meats is the tendency of PUFA to oxidize and reduce meat shelf life due to the rancidity and color deterioration. Meat with high levels of PUFA can rapidly oxidize, showing rancidity and color deterioration. A good way to avoid such problems is to use antioxidants products (such as vitamin E) in the diet. Some herbs and spices (rosemary, sage, clove, etc.) can be efficient food ingredients in improving the shelf life of meats vulnerable to oxidative changes because they contain many natural antioxidants (Zhang et al. 2010).” (Cheung & Mehta, 2015)
“Grass-based diets have also been shown to enhance CLA isomers, trans-vaccenic acid (C-18:1 t11), a precursor to CLA, and n-3 fatty acids in meat from ruminants. Grass-based diets also allow the production of beef meat with higher contents of precursors for vitamin A and E and other antioxidants such as glutathione and superoxide dismutase activity than grain-fed diets (Daley et al. 2010). In monogastric animals, it has also been observed that meat obtained from animals reared in a free-range system is rich in unsaturated fatty acids and antioxidants.” (Cheung & Mehta, 2015)
“Although the diet is the most important factor that modifies the fatty acid composition of animals, biotechnology is a future way to improve the fatty acid composition of meat lipids. Nowadays, there are transgenic pigs that express a plant gene encoding an n-3 fatty acid desaturase, and they can produce meat with high levels of n-3 PUFA and reduce n-6/n-3 PUFA ratio (Jime´nez-Colmenero et al. 2012).” (Cheung & Mehta, 2015)
“Meat color can be affected by a variety of factors, including postmortem handling, chilling, storage, and packaging. Besides, myoglobin content increases with age, e.g., veal is brownish pink, while beef from 3-year-old steers is bright, cherry red (James and James 2010).” (Cheung & Mehta, 2015)
Mechanically Recovered Meat
“Mechanically recovered, separated, or deboned meat is the meat obtained by the application of mechanical forces (pressure and/or shear) to bones from pork, beef, sheep, or goat or from poultry carcasses, whose meat has previously been manually removed. The mechanical process of removing meat from bones produces several changes in structure and composition of meat; the process causes cell breakage, protein denaturation, and increases in lipid and heme contents, and the meat has a pasty structure and poorer mechanical properties than manually deboned meat. Mechanically recovered meat (MRM) is mainly used in comminuted meat product formulation because of its texture and low cost compared with other meats although it is also incorporated to nonemulsified meat products in lower proportion. Its proteins show good gelling properties.” (Cheung & Mehta, 2015)
“The chemical composition of MRM depends on the species (Table 4) and age of animals, the proportion of bone and fat of the raw material, and the type of machine and the operating conditions applied.” (Cheung & Mehta, 2015)
“MRM is a fine ground, paste-like product due to the great fragmentation of myofibrils, with breaks in the Z or M bands. The shearing process modifies the length of fibrils and results in spherical to oval particles.” (Cheung & Mehta, 2015)
“Depending on species and anatomical part of the material being deboned, the protein content varies from 11.4% to 20.6%. The protein content in MRM is higher than in hand-boned meat mainly due to the incorporation of collagen during extraction.” (Cheung & Mehta, 2015)

“Lipid content of MRM is also higher than that of manually deboned meat because of the inclusion of the skin of abdominal fat, but fat mainly comes from the bone marrow and bone tissue. This fat is rich in PUFA due to the incorporation of phospholipids from the bone marrow; unsaturated fatty acids are considered as beneficial to health, although they are more prone to autoxidation which produces losses in meat sensory quality during storage. Cholesterol content is higher in MRM than in hand-boned meats. It is mainly released from the bone marrow, but can also be affected by the fat content and the presence of skin.” (Cheung & Mehta, 2015)
“The incorporation of bone particles and powdered bone during mechanical deboning cannot be avoided, and this increases ash and calcium contents to levels much higher than those of hand-boned meat. The calcium content varies depending on the species, bone type, feeding and age of the animals, and also the system used for deboning (pressure force machines increase calcium content due to the incorporation of higher amounts of bone particles).” (Cheung & Mehta, 2015)
“Iron content is two to three times higher in MRMthan in manually deboned meat because the red marrow hemoproteins are incorporated to it during pressing. MRM contains two to three times more hemoglobin, with no change in myoglobin content. This fact modifies meat color (is redder) and makes MRM even more susceptible to lipid oxidation.” (Cheung & Mehta, 2015)
“MRM is used in the manufacture of Bologna and mortadella sausages, frankfurters, patties, and hamburgers (Field 2004; Viuda-Martos et al. 2012).” (Cheung & Mehta, 2015)
Enhanced Meat and Comminuted and Reformed Fresh Meat Products
“Some meat products are formed by fresh, raw meat and other ingredients and do not suffer heat or drying treatment and, in many cases, not even curing. These products can be classified, according to the structural integrity of the meat used in their manufacture, into whole muscle and comminuted products.” a sausages, frankfurters, patties, and hamburgers (Field 2004; Viuda-Martos et al. 2012).” (Cheung & Mehta, 2015)
“Enhanced meat manufacture involves the addition of an aqueous solution, by pumping or injecting, to fresh whole meat cuts to improve juiciness and tenderness. The solution usually contains salt, phosphates dissolved in water, and sometimes other ingredients may be used: organic acids (lactic and citric acids) and antioxidants for improving shelf life and flavor and flavorings and flavor enhancers (spices, monosodium glutamate) and tenderizers (proteases such as papain, ficin, or bromelain) that hydrolyze muscle fibers and connective tissue.” (Cheung & Mehta, 2015)
“Fresh comminuted meats include ground beef, pork and poultry, nuggets, restructured patties, steaks, and chops, among others. These products are uncured, typically uncooked (although nowadays they can be found in the market full cooked and ready to use), and unseasoned but may include limited seasoning and/or binders.” (Cheung & Mehta, 2015)
“Fresh sausages (pork and beef sausages) are uncured ground products, seasoned with salt (usually about 0.5–1.0 %), sweeteners, and spices, usually stuffed into casings and not smoked or cooked. They do not contain curing salts (nitrate, nitrite) or phosphates. Fresh pork sausage typically has a high fat content (about 50 % fat) and contains antioxidants (butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate, rosemary extract). Fresh beef sausages usually have lower fat content (around 30 % fat).” (Cheung & Mehta, 2015)
“Nugget and restructured products may also be coated with batter or breading. In this case, some ingredients that help with batter adhesion are added: flour, starches, hydrocolloids, proteins, etc. (Sebranek 2003; Maddock 2012).” (Cheung & Mehta, 2015)
“Restructured meat products, or restructured whole-tissue meats, are manufactured by binding together pieces of meat of different sizes (from whole muscles to small particles) to give the final impression of a large meat cut that can be sliced into fillets or steaks, or cooked as a whole, resembling high-priced cuts such as a ham, a beef roast, or a turkey breast. They can be commercialized as raw (refrigerated or frozen) or cooked products. Restructured meats show lower cooking losses; uniformity of color, texture, and fat distribution; and the possibility of programming their nutritional value. They allow for the use of cheap meat cuts.” (Cheung & Mehta, 2015)
“Salt and alkaline phosphates are added to meat pieces in appropriate levels and, combined with mechanical action, result in the extraction of myofibrillar proteins to form a surface protein matrix that binds the pieces and the particles of fat. There are two methods of restructuring, cold-set binding and hot-set binding. In hot-set restructuring, a heat set gel is formed from extracted myofibrillar protein which stabilizes particle binding and also retains water and lipids. The surface-binding matrix can also be created by the addition of other binders such as starches and proteins (whey, soy, blood plasma and egg proteins, caseins, gluten, gelatin).” (Cheung & Mehta, 2015)
“Cold-set techniques include the use of alginate, fibrinogen and thrombin, and transglutaminase to form gels that bind the meat pieces. Transglutaminase is an enzyme that catalyzes an acyl transfer reaction between the γ-carboxylamide group of a peptide-bound residue and a primary amine; in restructured meat, the enzyme catalyzes the formation of chemical cross-links between glutamine and lysine residues of protein chains. It is a fast, cold-gelling meat binder that produces restructured meats with high physical strength and thermal stability. Commercial preparations contain transglutaminase of microbial origin in concentrations of 1 %. It can develop its action at temperature of refrigeration, although its optimum conditions are around 55 oC and pH 6–7; it is used in conjunction with sodium caseinate, sugar, fatty acid ester, and dextrin or with sodium polyphosphate, sodium pyrophosphate, ascorbate, and lactose (Farouk 2010). Table 5 shows the chemical composition of comminuted, enhanced, and reformed meat products.” (Cheung & Mehta, 2015)”
Cooked-Cured Meat Products
“Cooked-cured meat products are products manufactured with the addition of curing agents and submitted to heat treatment. They are classified into two types according to their structure: emulsion-based products and products from whole meat cuts.” (Cheung & Mehta, 2015)
Heat treatment modifies the structure of meat proteins. Most of the sarcoplasmic proteins aggregate at 40–60 oC, although in some cases the coagulation can prolong up to 90 oC. Myofibrillar protein unfolding starts at about 30 oC, followed by protein-protein associations at 36–40 oC and consequential gelation at 45–50 oC. Collagen denaturation occurs between 53 oC and 63 oC, followed by collagen fiber shrinkage. If collagen fibers are not stabilized by heat-resistant intermolecular bonds, it forms gelatin on further heating (Tornberg 2005).” (Cheung & Mehta, 2015)
Emulsion-Based Meat Products
“Emulsion-based meat products, or meat batters, are products based in a complex matrix in which fat is emulsified into a viscous fluid mainly composed of solubilized myofibrillar proteins previously extracted from meat and finally stabilized by a heat treatment that produces gel formation. In these products, meat particles are so small that they are not visually distinguishable on the product surface. Examples of these products are frankfurters, wieners, bologna sausage, mortadella, fine liver sausages, and liver pate. Pork and beef are the most common meats used in these products, although the usage of poultry meat is increasing in the last years due to its nutritional characteristics. Mechanically recovered meat is frequently used in the formulations, and, specifically, liver is one of the main raw materials for liver sausages and pate.” (Cheung & Mehta, 2015)
“An emulsion consists of two immiscible liquids (usually water and fat in food emulsions) in which one of the liquids is dispersed as small droplets (dispersed phase) in the other (continuous phase). Meat emulsions, however, are much more complex systems: the dispersed phase is itself a multiphase media that contains solid fat particles (1–50 μm in size), liquid fat droplets, and air bubbles. The continuous phase is constituted by water and many other compounds, such as proteins, salt, carbohydrates and insoluble proteins, connective tissue, and meat particles.” (Cheung & Mehta, 2015)
“According to the processing method, there are two types of emulsified meat products: cold emulsions (e.g. frankfurters) and hot emulsions (e.g. pates). In cold emulsions obtaining, the ingredients are raw (uncooked) when they are finely comminuted and the emulsion is formed. In hot emulsions, some raw materials are precooked before emulsification. The resulting viscous batter is portioned or stuffed into casings and submitted to heat treatment.” (Cheung & Mehta, 2015)
“In meat emulsions, the main function of sodium chloride is to release the myofibrillar proteins and to increase their ability to emulsify fat, specially at pH values near the pI. Both myofibrillar and sarcoplasmic proteins can act as emulsifiers, although myofibrillar proteins are preferably absorbed to the water/fat interface. Myosin is more surface active than actin or actomyosin. Protein hydrophobicity is important for effective formation of the interfacial protein film that surrounds fat globules and stabilization of the protein matrix. Hydrogen bonds and electrostatic attractions appear to have some importance, participating in interfacial film-protein matrix binding.” (Cheung & Mehta, 2015)
“Salt also contributes to improve the water retention of myofibrillar proteins by the mechanisms described above (section “Water”); this is a very important characteristic that helps the sensory properties of these products.” (Cheung & Mehta, 2015)
“Mild warming during grinding and mixing for emulsion formation aids in solubilization and releasing of myofibrillar proteins, but temperatures above 22 oC may cause emulsion breakdown due to protein denaturation before they can act as emulsifiers and orientate in the water-lipid interface.” (Cheung & Mehta, 2015)
“The system is stabilized by protein denaturation during heat treatment, due to the strong gel produced by myofibrillar proteins; sarcoplasmic proteins produce very weak gels and they do not contribute significantly to stabilization and structure formation. During heating, myofibrillar proteins start to denature, exposing hydrophobic domains. This makes the formation of hydrophobic interactions more probable; protein aggregation is enhanced and immobilizes the fat globules by physical entrapment (Lawrie 1998; Ugalde-Benı´tez 2012).” (Cheung & Mehta, 2015)
Conclusion
An overview by anybody involved in meat processing of the basic structure and function of meat is of the greatest importance in order to gain insight into our trade. The fact that particle size reduced to too small particles negatively impacts the functional characteristics of meat is clear. Certain processing techniques damage the cell structure too much and the cell loses structure. There are, of course, instances where the cell must be opened up as far as possible to access certain cell constituents and such very fine comminution is exactly what is required. The purpose for ultra-fine comminution must therefore be well understood before it is attempted.
Further Reading
Evaluation of quality characteristics of chicken meat emulsion/nuggets prepared by using different equipment
References
Cheung P. C. K., Mehta, B. (Editors). 2015. Handbook of Food Chemistry. Springer.
Lewis, L., Downes, M. N.. 2019. Chapter 6: The Musculature of the Rat. From Anatomy and Histology of the Laboratory Rat in Toxicology and Biomedical Research. Pages 57-76
Listrat, A., Lebret, B., Louveau, I., Astruc, T., Bonnet, M., Lefaucheur, L., Picard, B., Bugeon, J.. 2016 “How Muscle Structure and Composition Influence Meat and Flesh Quality”, The Scientific World Journal, vol. 2016, Article ID 3182746, 14 pages, 2016. https://doi.org/10.1155/2016/3182746
Purslow P.. 2020. The Structure and Role of Intramuscular Connective Tissue in Muscle Function, Frontiers in Physiology 11, 2020, https://www.frontiersin.org/article/10.3389/fphys.2020.00495, DOI=10.3389/fphys.2020.00495, ISSN=1664-042X
Voutila, L. 2009. Properties of intramuscular connective tissue in pork and poultry with reference to weakening of structure. Helsinki University Print.

